Compressibility factor z versus 100/V, for several values of Pressure

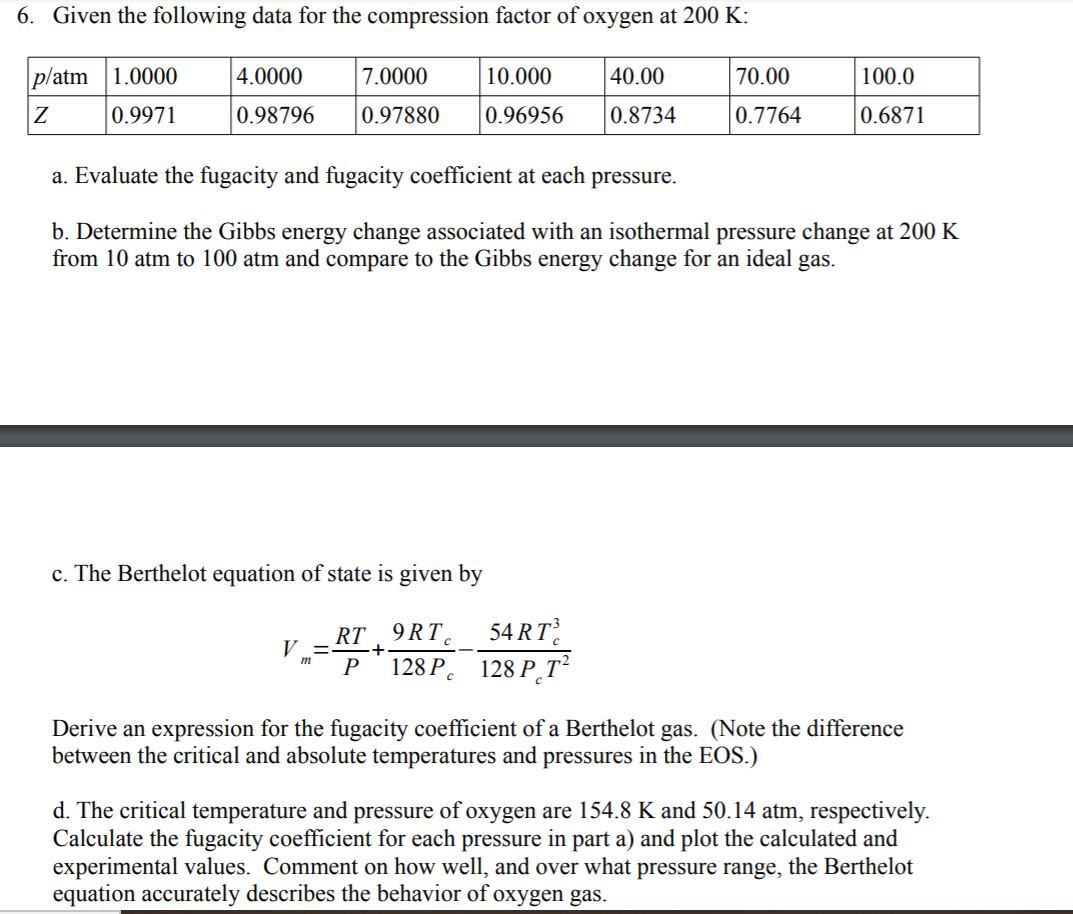
6. Given the following data for the compression

A : At high pressure , the compressibility factor Z is (1 + (pb)/(RT))

Luis GONZÁLEZ-PORTILLO, PhD - Associate Professor

the compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0.5
Real Gases and the Virial Equation

PDF) Thermodynamic mapping of power cycles working around the
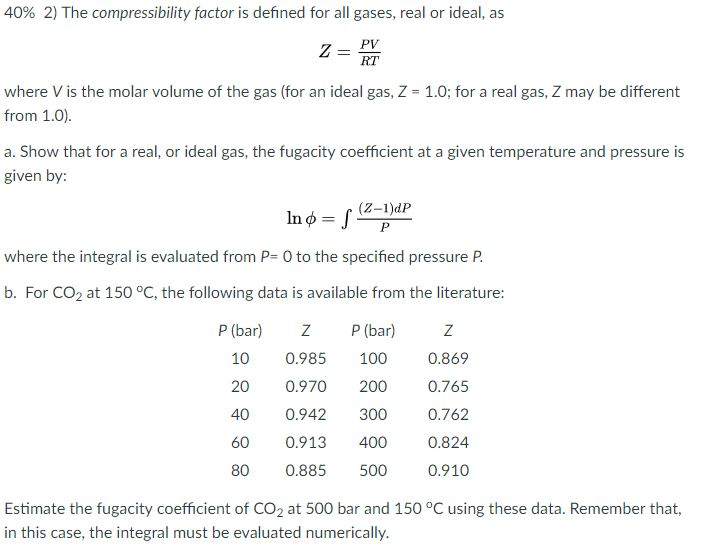
Solved 40% 2) The compressibility factor is defined for all

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

Phase transformation path in Aluminum under ramp compression; simulation and experimental study

Jose MARTINEZ-VAL, Chair Professor of Thermal Engineering

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas

Non-Ideal Gas Behavior Chemistry: Atoms First