42g of N₂ react with excess of O₂ to produce NO. Amount of NO

Description
Share your videos with friends, family, and the world

42g of N₂ react with excess of O₂ to produce NO. Amount of NO formed is a.60g b.32g c.45g d.90g
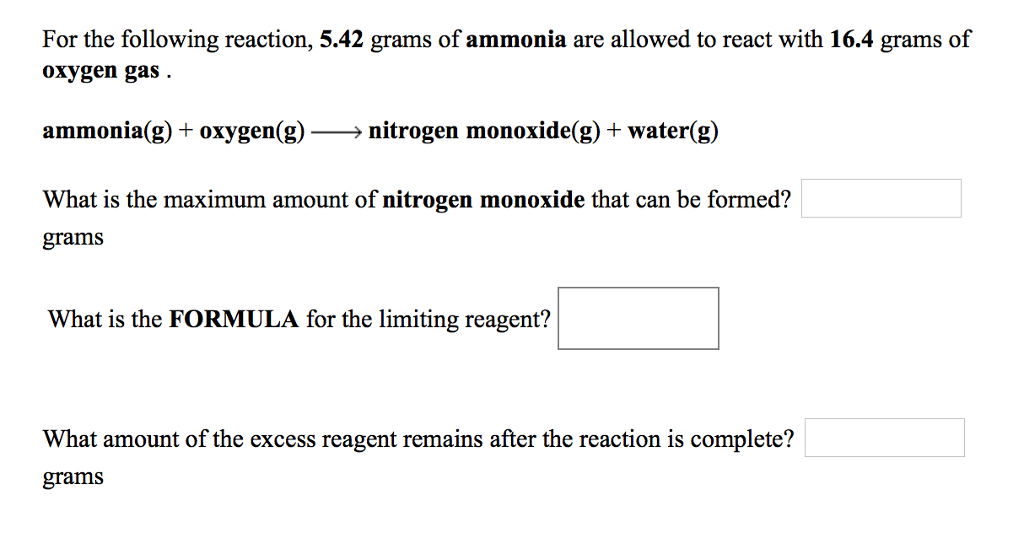
Solved For the following reaction, 5.42 grams of ammonia are
If 25 grams of CO reacted with 6.00 grams of H2, which is the limiting reactant and theoretically yield of CH3OH? - Quora
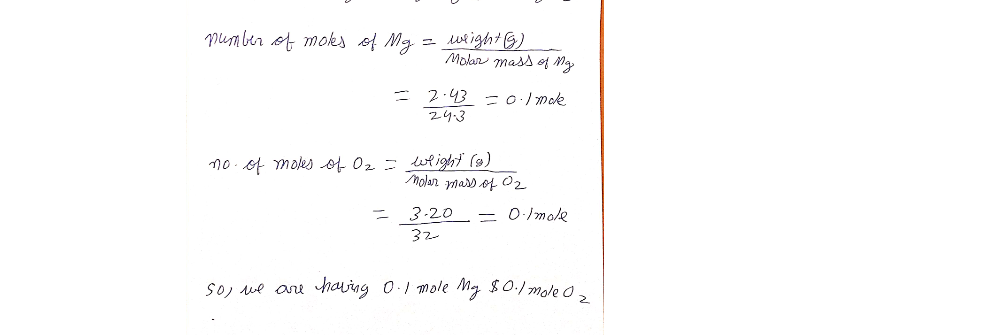
Answered: Suppose 2.43 g of magnesium is reacted…

Solved 21. Consider the following chemical reaction: N2+ O2
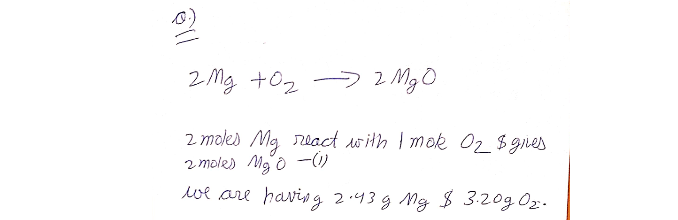
Answered: Suppose 2.43 g of magnesium is reacted…
How many moles of N2 are needed to produce 42.4 moles NH3? - Quora
7693-52-9, 4-Bromo-2-nitrophenol

1-ChapteR-Short-Question-With-Answer (20 Files Merged) PDF
Related products

Kit c/16 Ana Maria Napolitano 42g - Bolo / Bolinho / Mini Bolo

Laroscorbine Palladium Gold Box Vitamin C 42G & Collagen 15Gram at Rs 5700/box, Skin Whitening Products in New Delhi
Chocolate Snickers Morango 42g c/20 - Mars - Doce Malu

C Elite High Protein Shake with 42g Protein by fairlife Milk, Chocolate, 14 fl oz, 12 count
$ 8.99USD
Score 4.7(389)
In stock
Continue to book
$ 8.99USD
Score 4.7(389)
In stock
Continue to book
©2018-2024, belizeairportsauthority.com, Inc. or its affiliates



