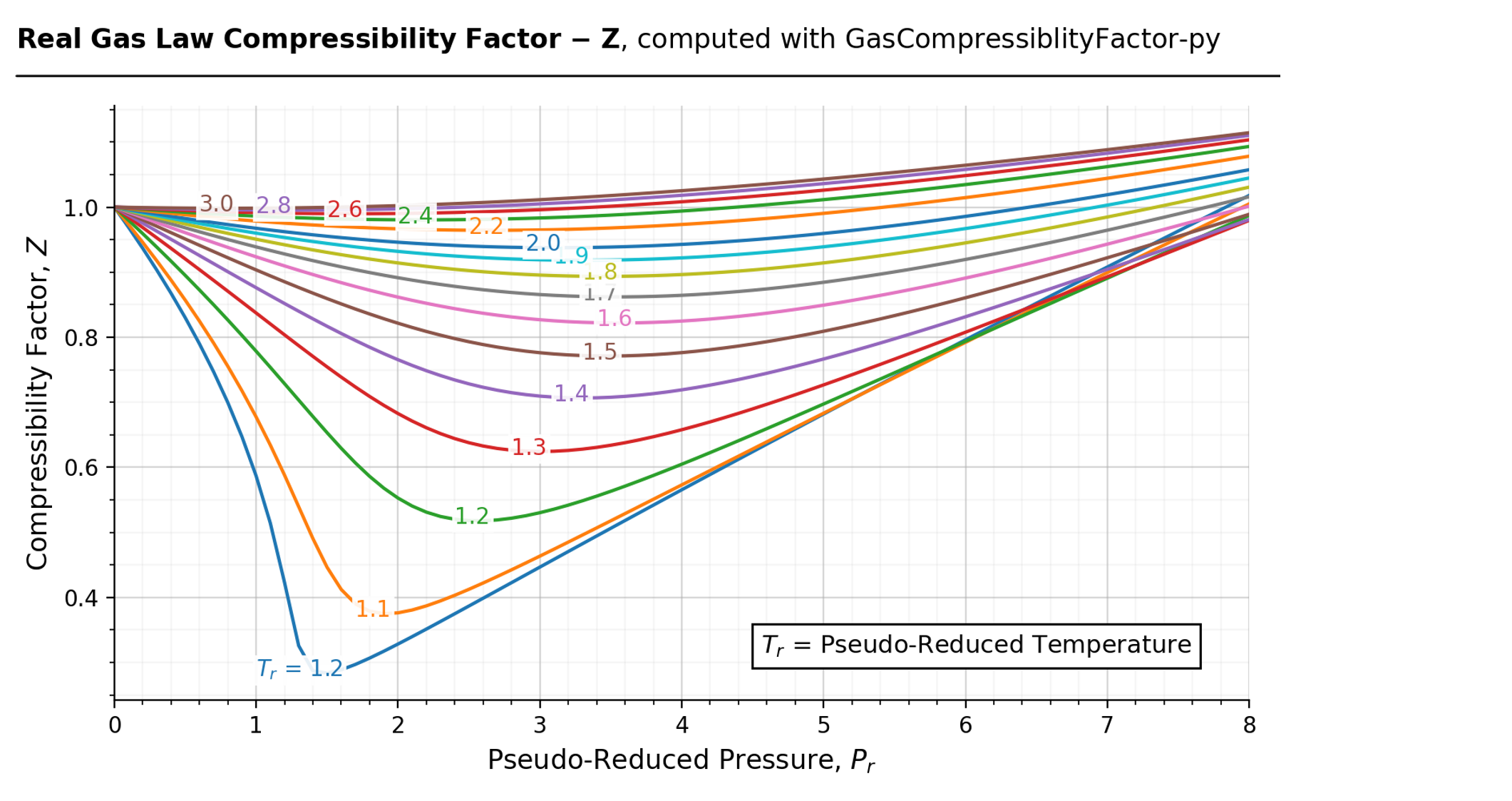
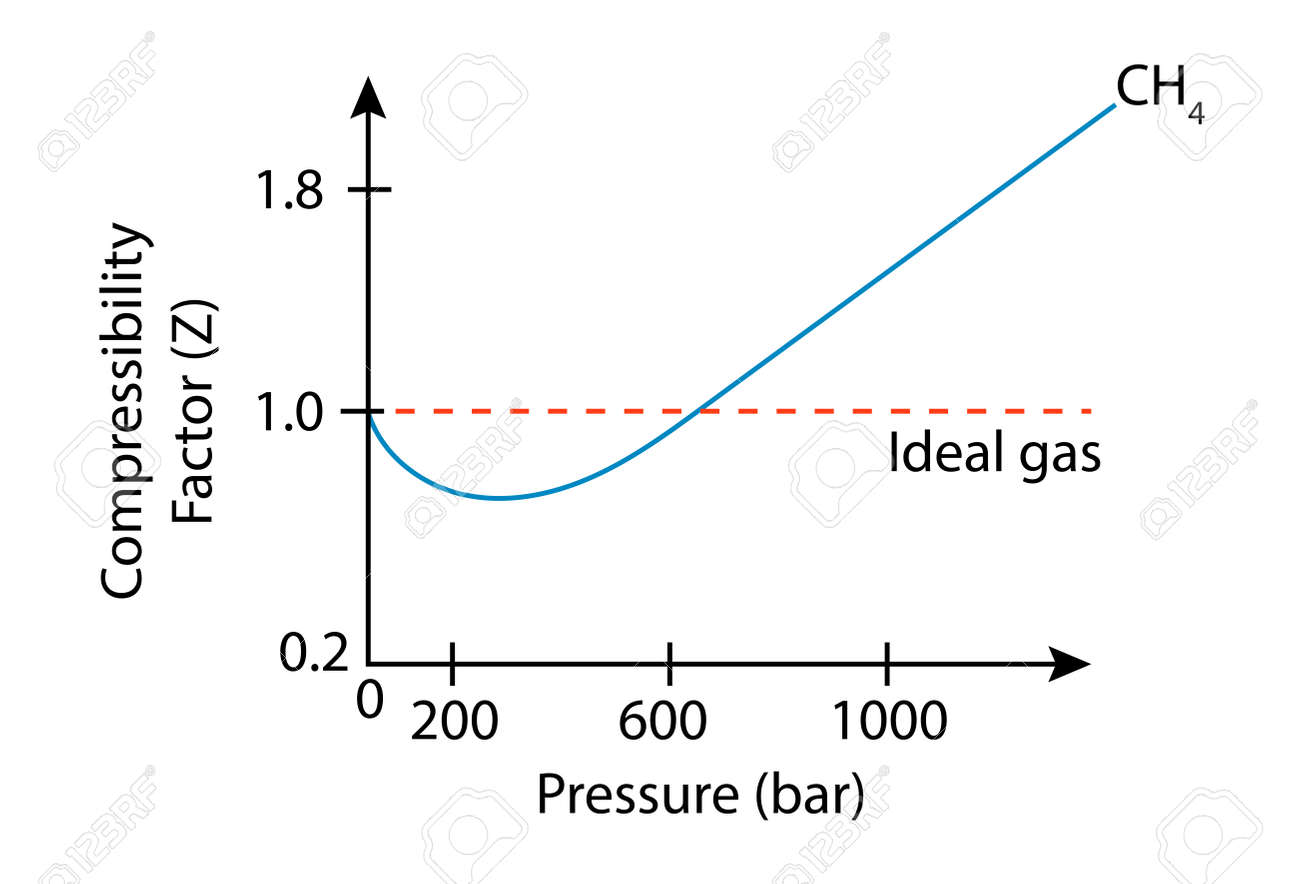
Compressibility factor (Z) for a van der Waals real gas at

Share your videos with friends, family and the world
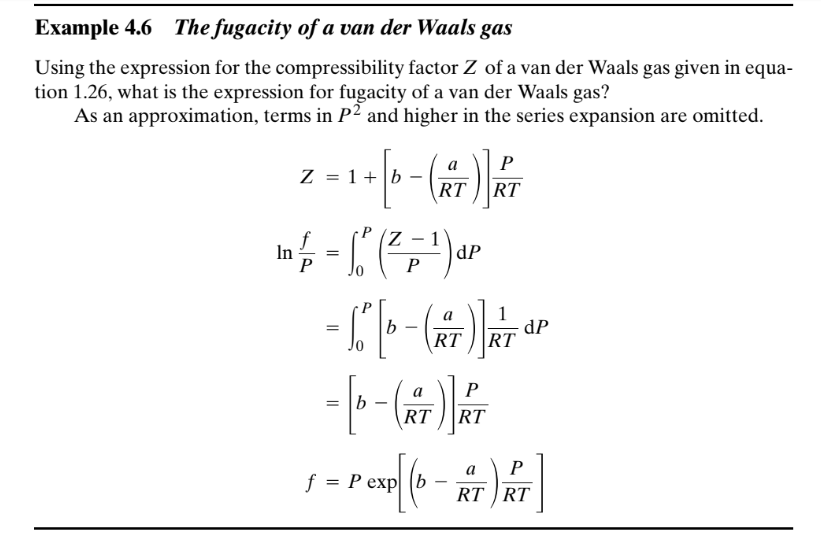
Solved Example 4.6 The fugacity of a van der Waals gas Using

Explain how the compression factor varies with pressure and
Complete Solutions to Mock Test 1 of chapter MOCK TEST of Class 11 book with complete answers and questions
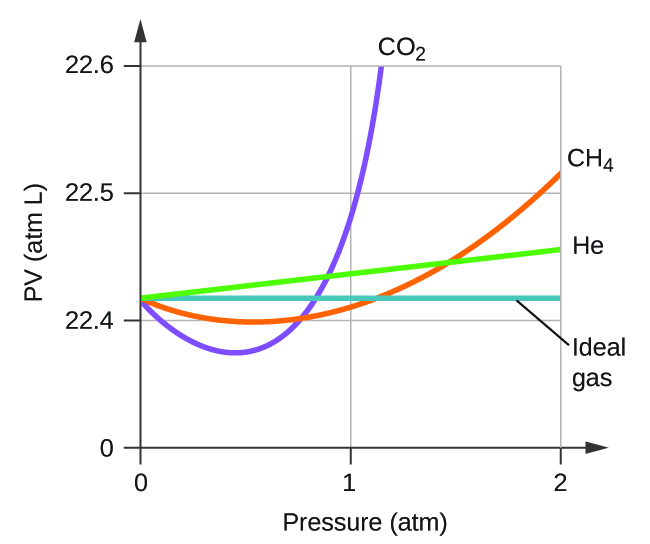
Non-Ideal Gas Behavior – Chemistry

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
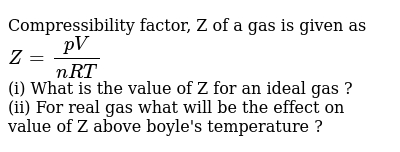
Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What
The value of compression factor at the critical state of a vander waals gas is

Deviations from ideal gas behaviour, intermolecular forces, Van der Waals equation of state, compressibility factors and the critical pressure and critical temperature of a gas revision notes doc brown's chemistry UK advanced
If Assertion is true statement but Reason is false, then mark (3)
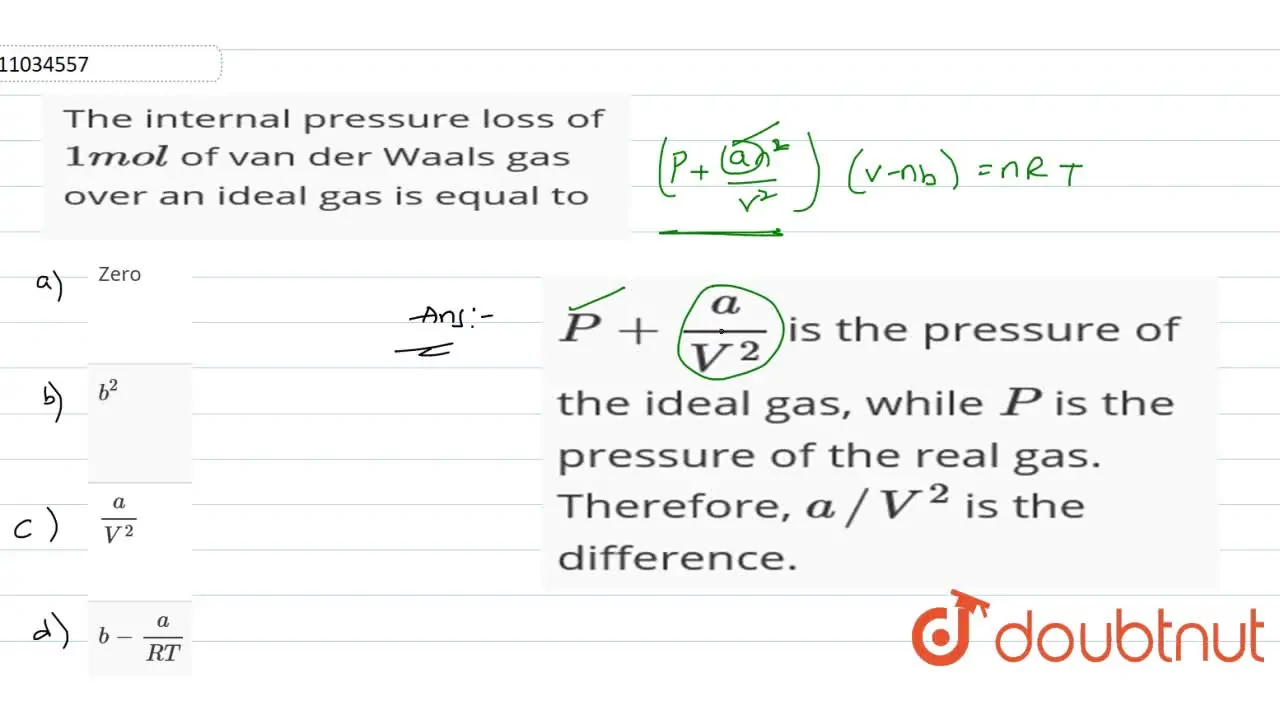
The internal pressure loss of 1 mol of van der Waals gas over an ideal

Solved 9 Compression factor Z Use the van-der-Waals equation
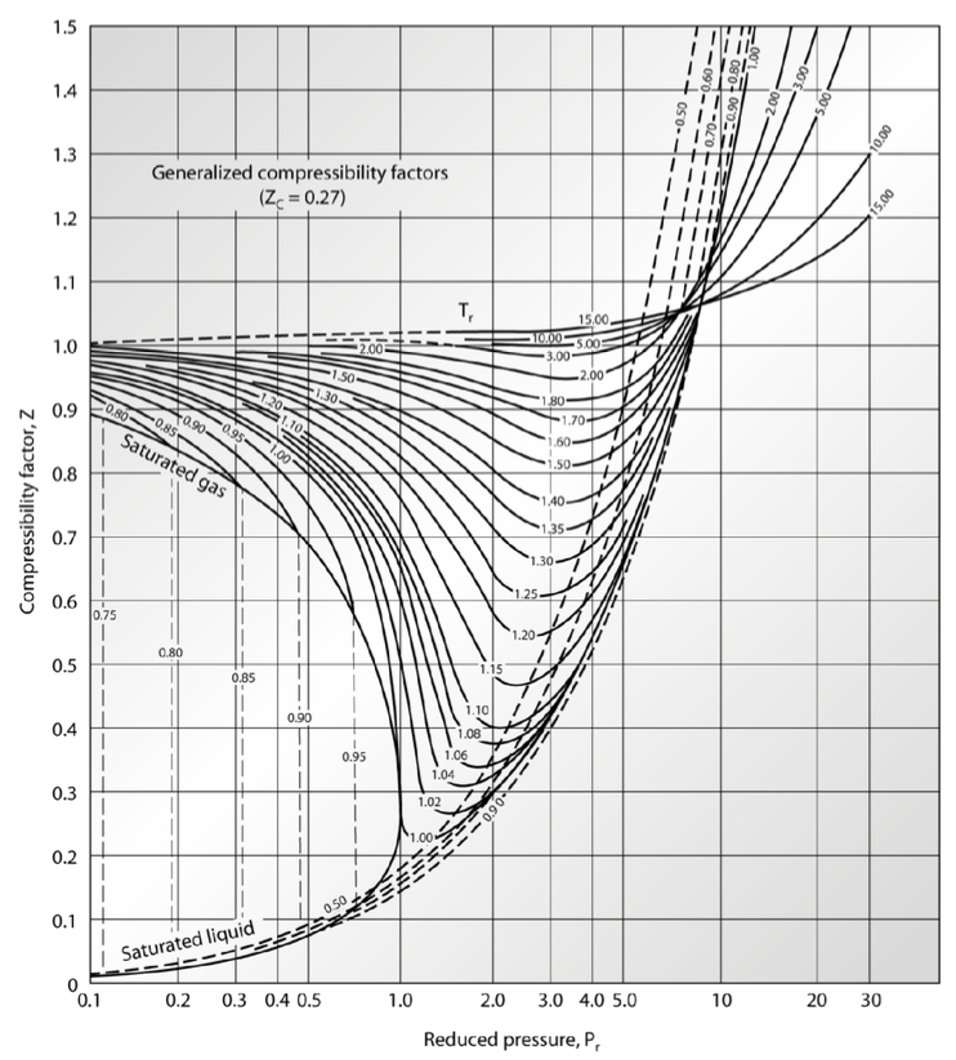
Thermo] Derivation of compressibility factor vs reduced pressure

Non Ideal Gas Behavior-chemistry - Non Ideal Gas Behavior Calculate the compressibility factor (Z) - Studocu
Qin Lab - thermal data