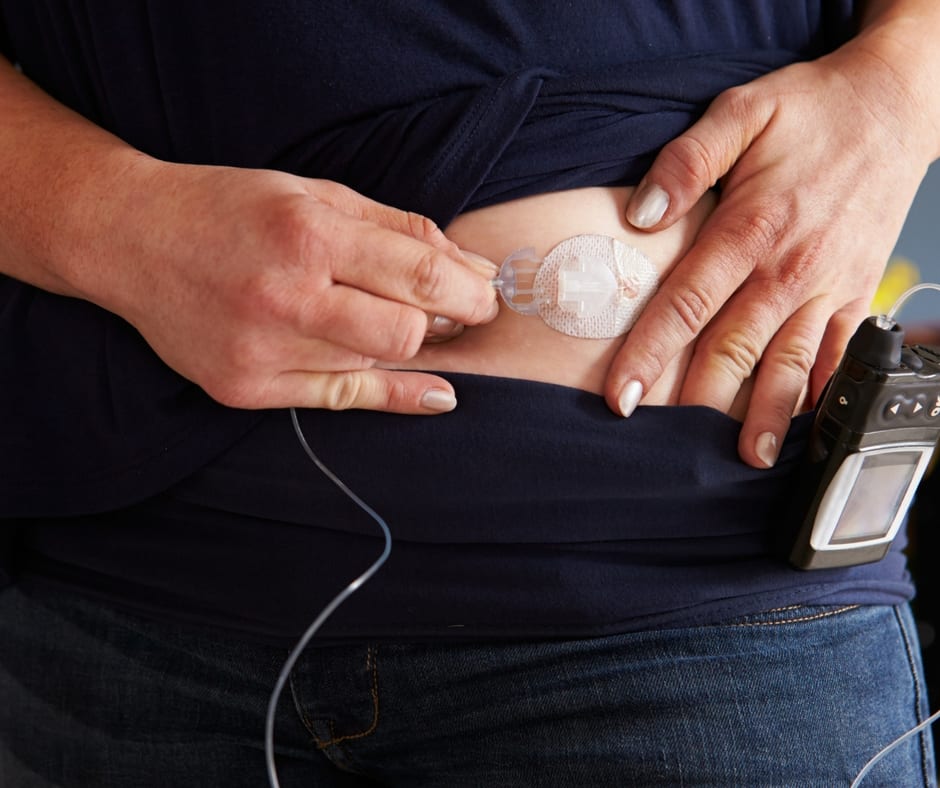
FDA says Medtronic MiniMed insulin pump recall is serious - MassDevice

The U.S. FDA has designated a recall of hundreds of thousands of Medtronic Minimed insulin pumps as Class I — the most serious type of recall. Medtronic (NYSE:MDT) first warned of safety problems with the pumps in November. The recall involves 322,005 pumps — MiniMed 630G (model MMT-1715) and MiniMed 670G (model MMT-1780) — in the […]

Medtronic expands 2 Class I recalls of MiniMed insulin pumps

Medtronic recalls some insulin pumps as FDA warns they can be hacked
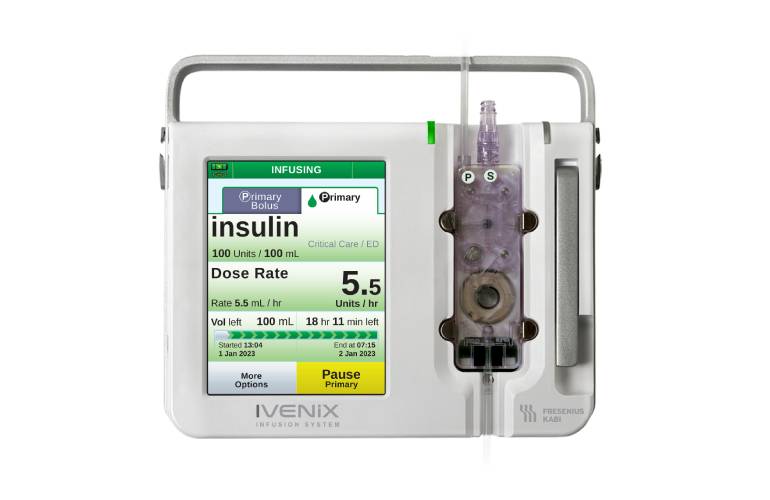
Fresenius Kabi has a Class I Ivenix infusion pump recall
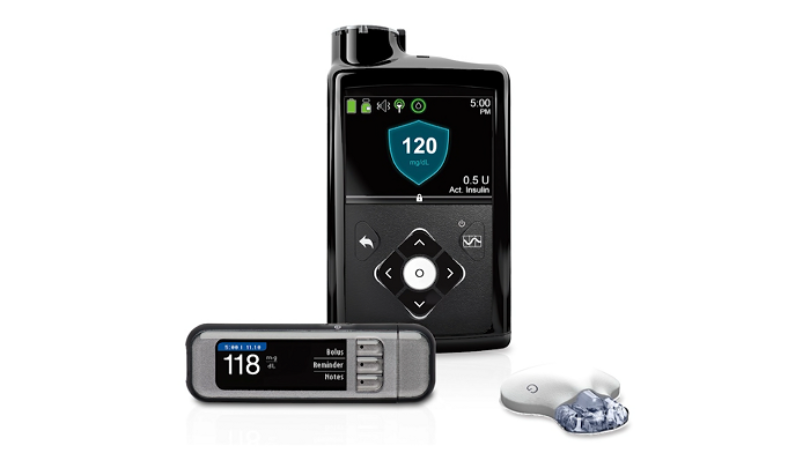
Medtronic MiniMed 600 Series Insulin Pumps Recalled
Medtronic Pain Pump SynchroMed II Blamed for Deaths

Medtronic MiniMed Insulin Pump Lawsuit
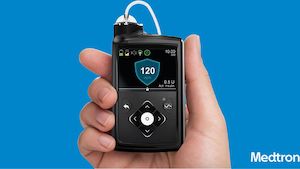
Medtronic Diabetes Pump Lawsuit - MiniMed Insulin Pump

Medtronic Diabetes Pump Lawsuit - MiniMed Insulin Pump

Chris Newmarker on LinkedIn: Data supports Medtronic MiniMed 780G insulin pump for children
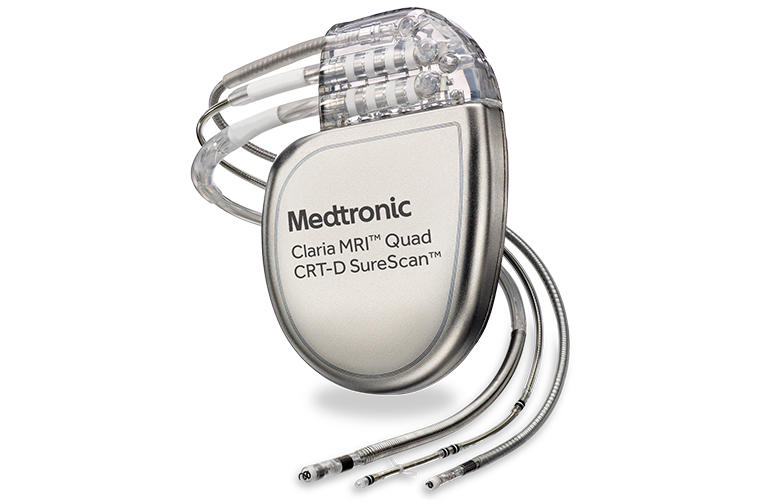
Medtronic's recalled ICDs and CRT-Ds are too risky to replace

Insulin pump recall: MiniMed pumps recalled by FDA

Medtronic recalls MiniMed insulin pumps as FDA warns about hacking

Medtronic MiniMed Insulin Pump Lawsuit March 2024 - Select Justice

Retail For Lease 7530 Lower Buckeye Rd, Phoenix AZ, 43% OFF
Medtronic MiniMed Insulin Pump Lawsuit & Recall