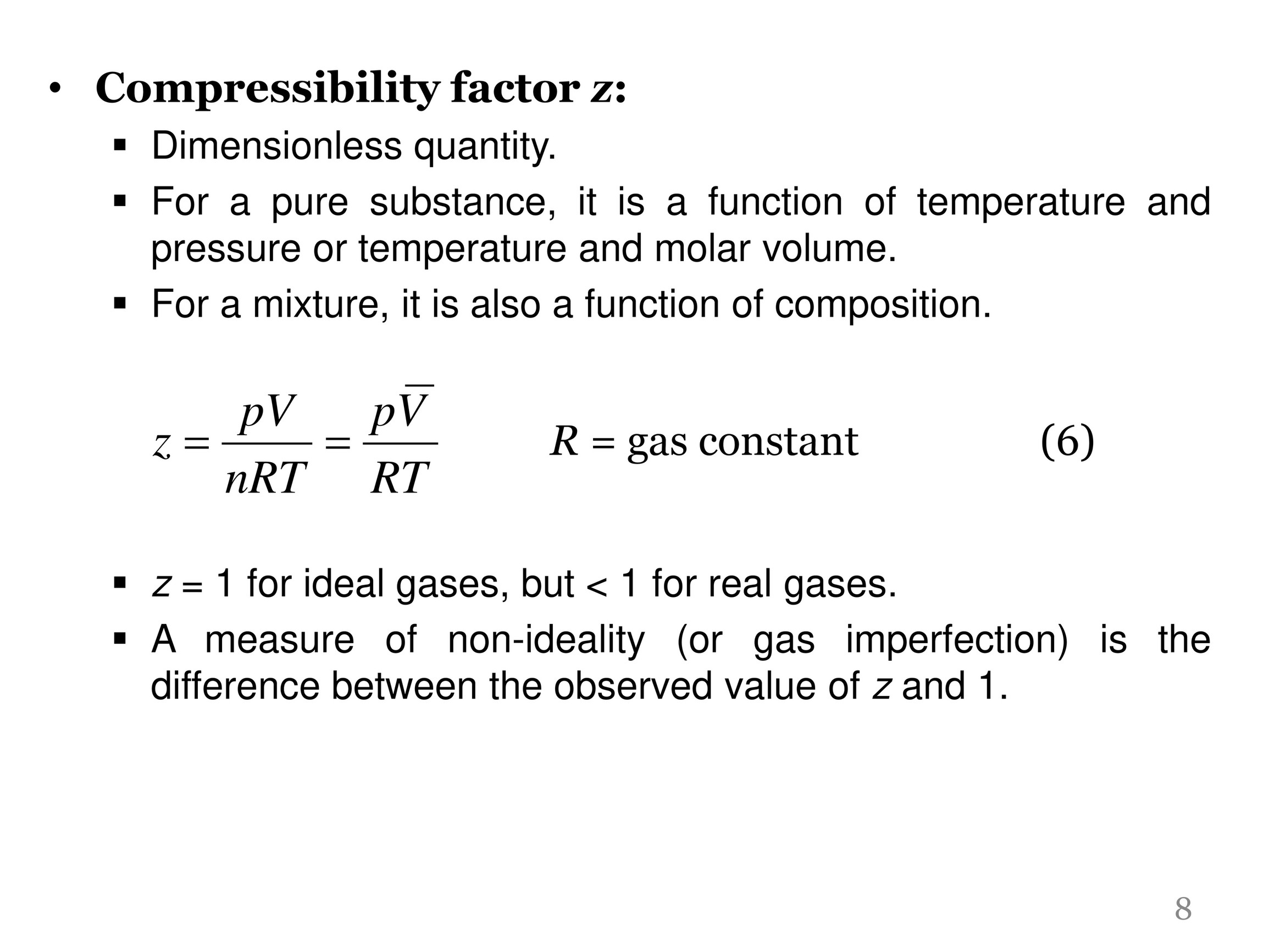
Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

Click here:point_up_2:to get an answer to your question :writing_hand:compressibility factor z of a gas is given as z frac pv nrt
Click here👆to get an answer to your question ✍️ Compressibility factor- Z of a gas is given as Z- frac - pV - nRT - -i- What is the value of Z an ideal gas-ii- For real gas what will be the effect on value of Z above Boyle temperature
Joule Thomson effect [JT]: A short review

Real gas z-Factor chart [2] Download Scientific Diagram

Compressibility factor - Wikipedia

Figure . Compressibility factor Z = PV/NkT of the SW fluid plotted

Compressibility Factor Calculator
Which gas shows the maximum deviation from ideal gas, CO2 or NH3? Why? - Quora

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

What is compressibility factor? What is its value for ideal gas

The given graph represents the variation of Z(compressibility factor =- PV nRT ) versus P, three real gases A, B and C. Identify the only incorrect statement. Ideal gas P (atm) (A)

Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What
My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created with Publitas.com

The compressibility factor `(Z=PV//nRT)` for `N_(2)` at `223 K` and `81.06 MPa` is `1

Energies, Free Full-Text