At high pressure, the compressibility factor 'Z' is equal toa


A : At high pressure , the compressibility factor Z is (1 + (pb)/(RT))

At critical temperature, pressure and volume. The compressibility factor (Z) is 2

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!
If Assertion is true statement but Reason is false, then mark (3)

Assertion: Compressibility factor for hydrogen varies with pressure wi

NEET Chemistry Chapter Wise Mock Test - Mock Test 2 - CBSE Tuts

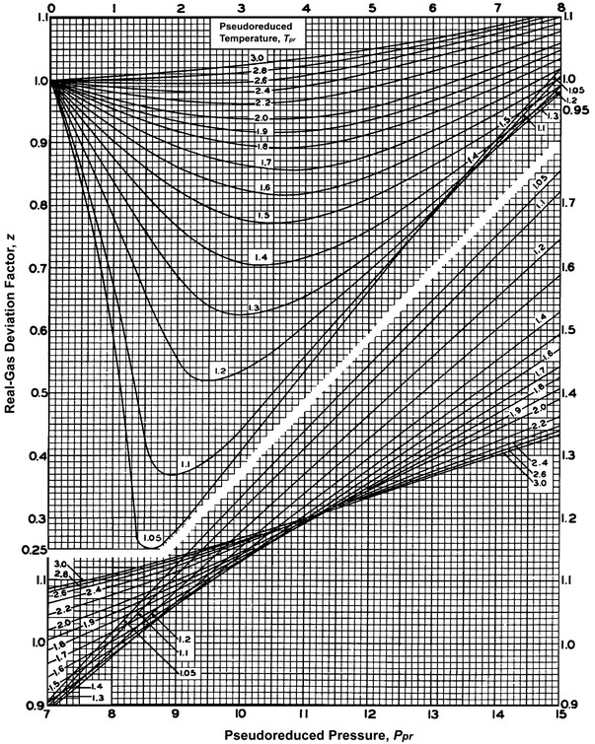
Compressibility factor Z

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

Compressibility factor Z versus density at several representative
What is the value of compressibility factor for a non-ideal gas? - Quora

Variation OF compressibility factor with pressure

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson