Compressibility factor (Z) for a van der Waals real gas at critical point is

Share your videos with friends, family and the world
Van der waals equation: Derivation, Explanation
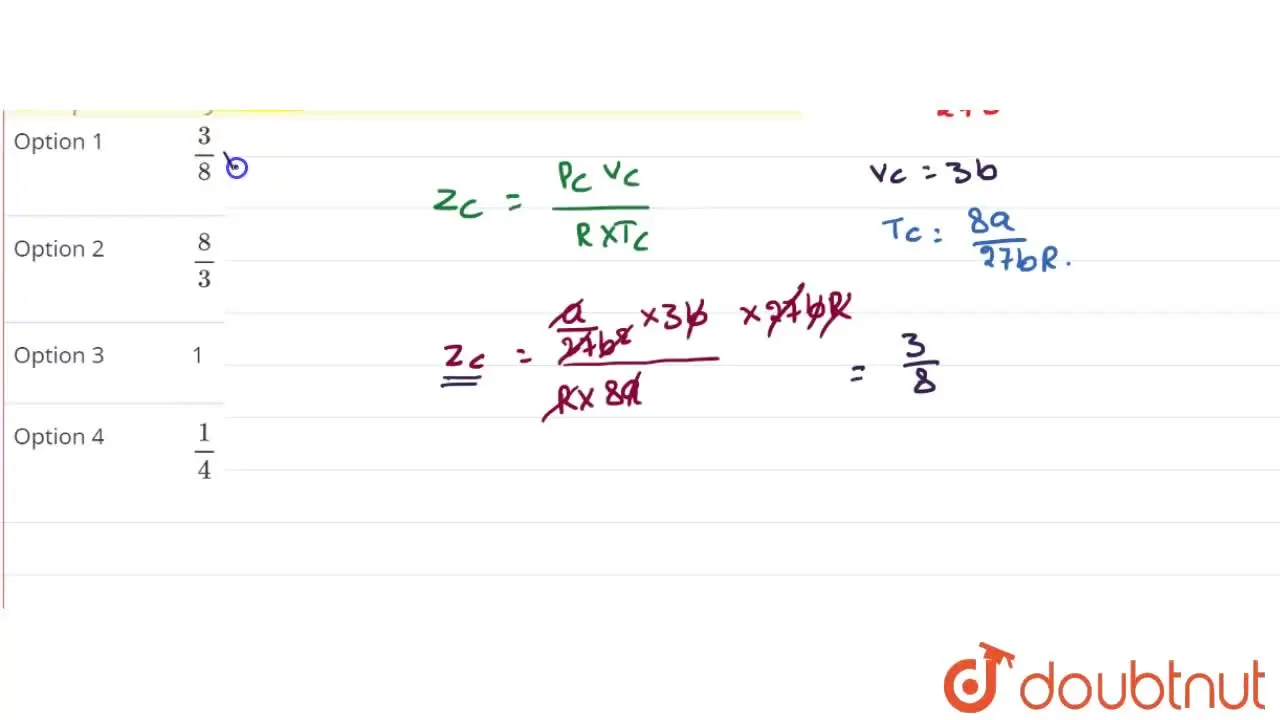
Telugu] Under critical states for one mole of a gas, compressibility

The van der Waals equation of state at the critical point

Basic Principle II Second Class Dr. Arkan Jasim Hadi, PDF, Gases
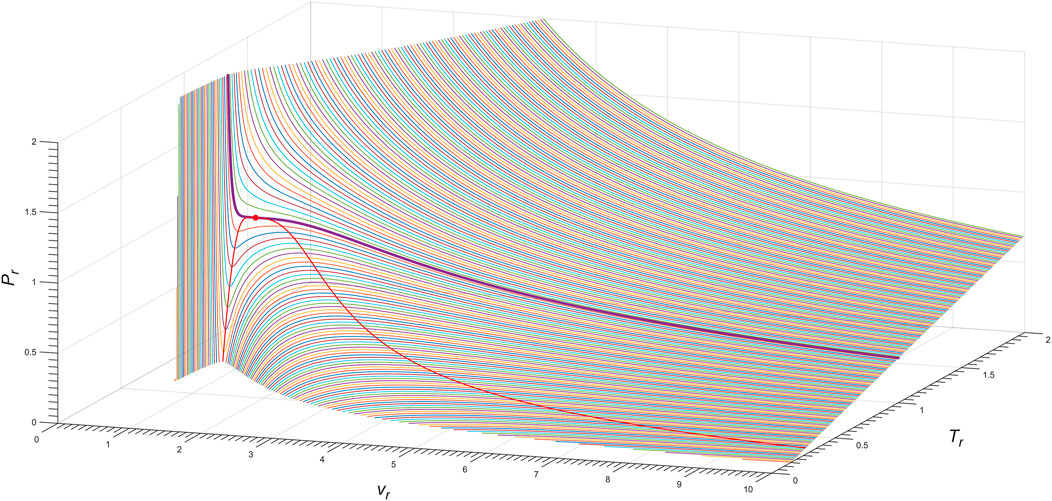
Frontiers Janus van der waals equations for real molecules with two-sided phase transitions
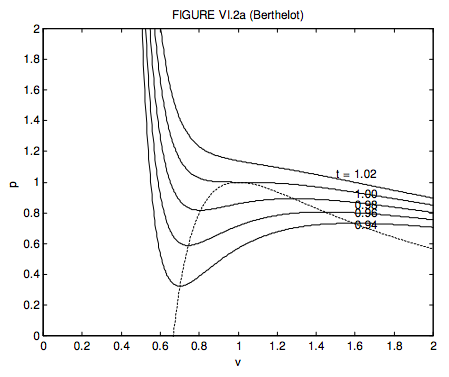
6.3: Van der Waals and Other Gases - Physics LibreTexts

Simple Equation Real Gas Compressibility Factor Z

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

Solved Problem 1. 15 points Find the compressibility factor
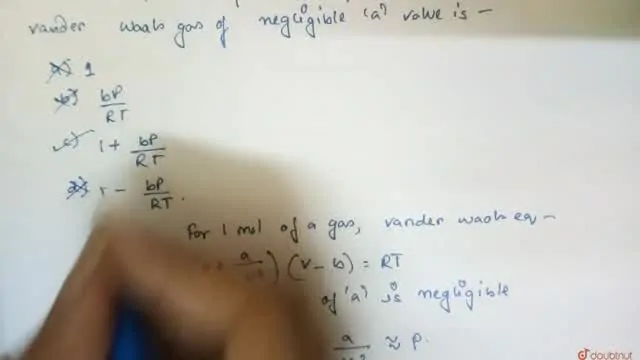
Bengali] The compresibility factor (Z) of one mole of a van der waals

Ideal Gas Equation - an overview

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

The compressiblity factor a gas obeying van der Waals' equation of state is given by V V-b RTV (2) a ✓ RTV V-b V-b RTV (3) Va (4) RTV V-6

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
Complete Solutions to Mock Test 1 of chapter MOCK TEST of Class 11 book with complete answers and questions