The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is -Enthalpy of vaporization for water is 40-63 kJ mol-1
36 g of water at 30°C are converted into steam at 250°C at constant atmospheric pressure. - Sarthaks eConnect
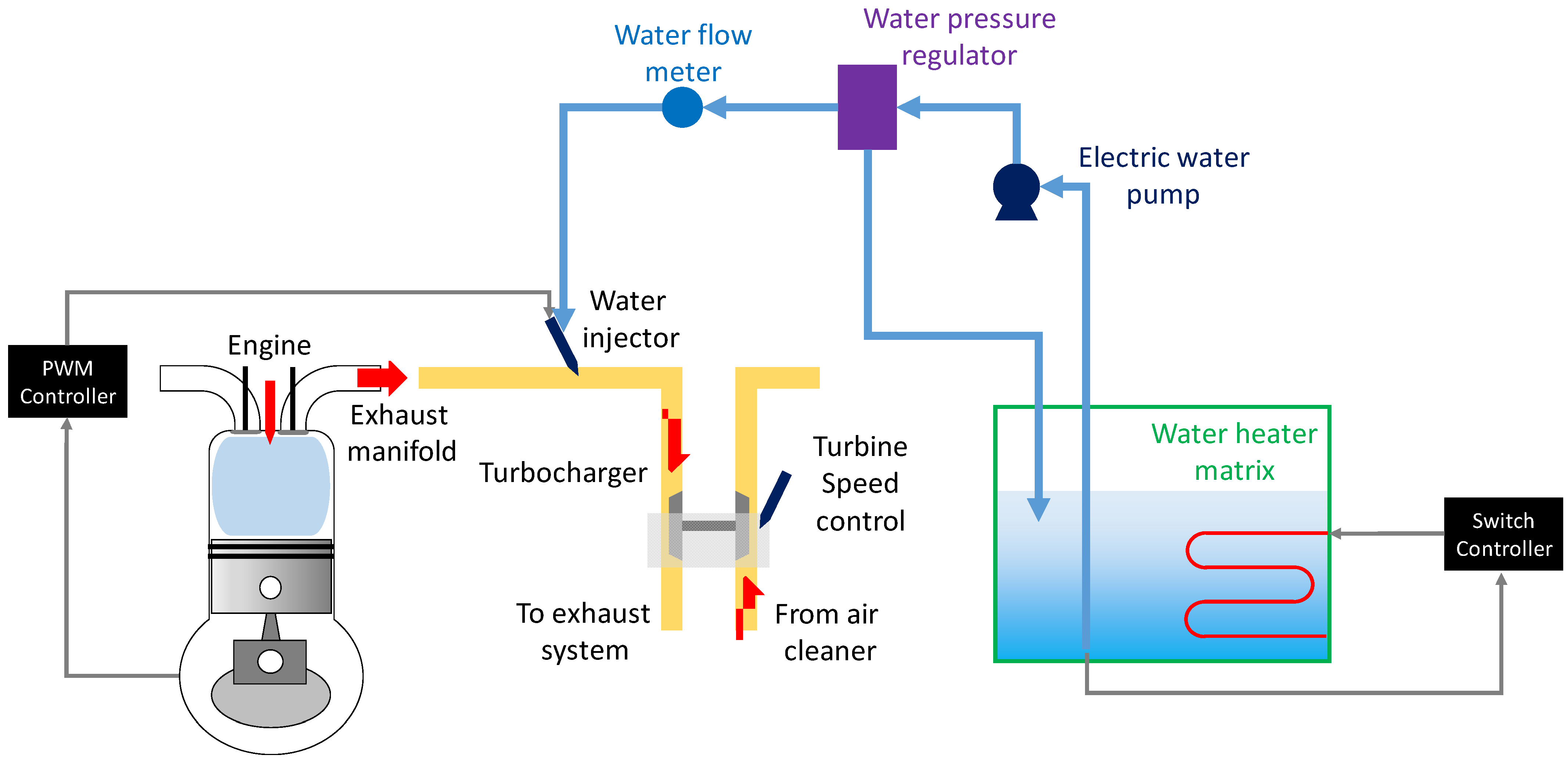
Energies, Free Full-Text
The free energy and entropy change in kJ per mole whenliquid water boils at 1 atmosphere are respectively(latent heat of water= 2.0723 kJ g^ 1) (a) 0, 0 (b) 0.1, 0.1 (c) 0.1,0 (d) 0, 0.1
The entropy change associated with the conversion of 1kg of ice at 273K to water vapours at 383K is: - Sarthaks eConnect

The entropy change associated with the conversion of 1 kg of ice 273 K to water vapours 383 Kis: (specific heat of water liquid and water vapour are 4.2 kJ K-kg- and
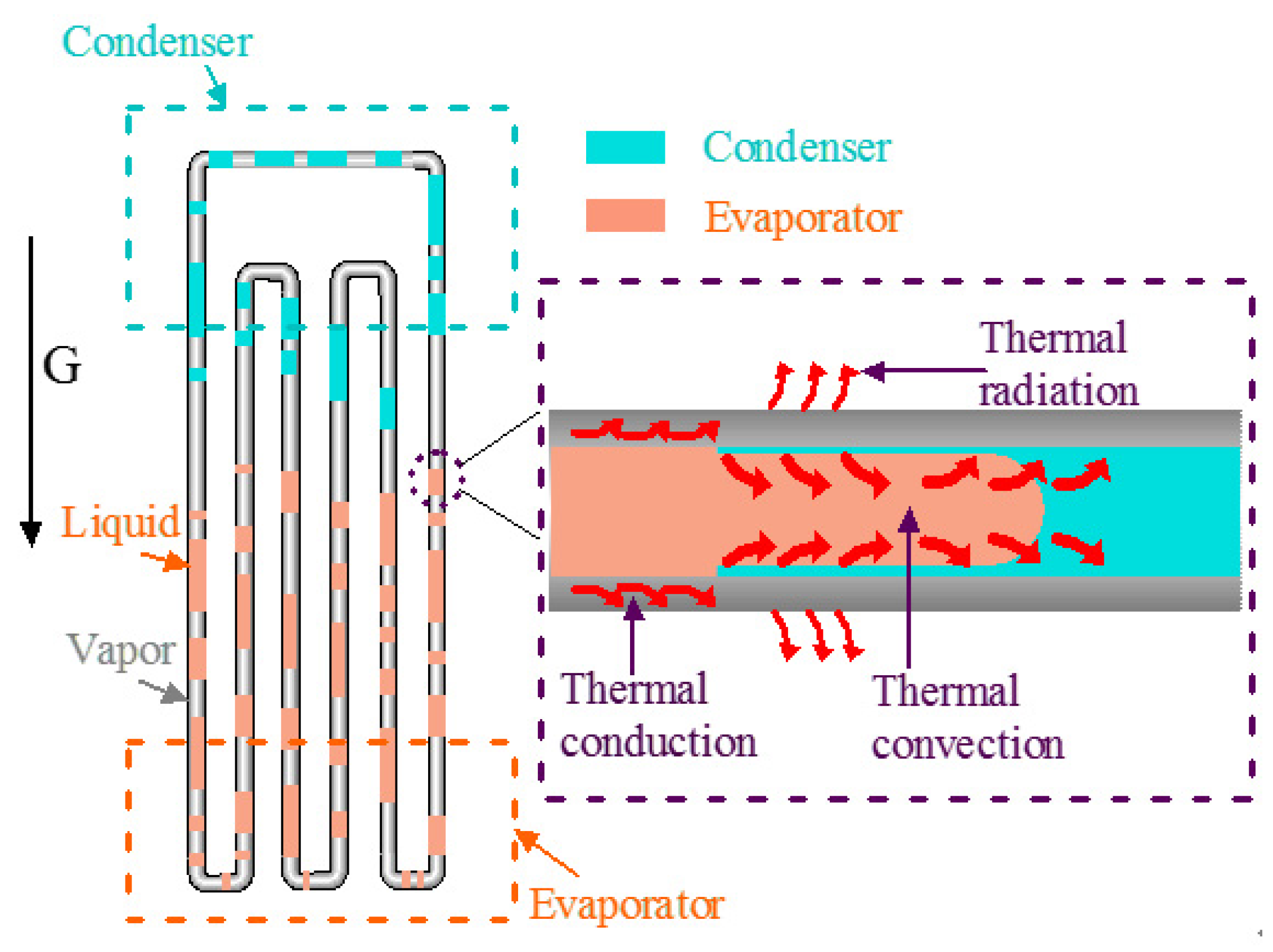
Sustainability, Free Full-Text
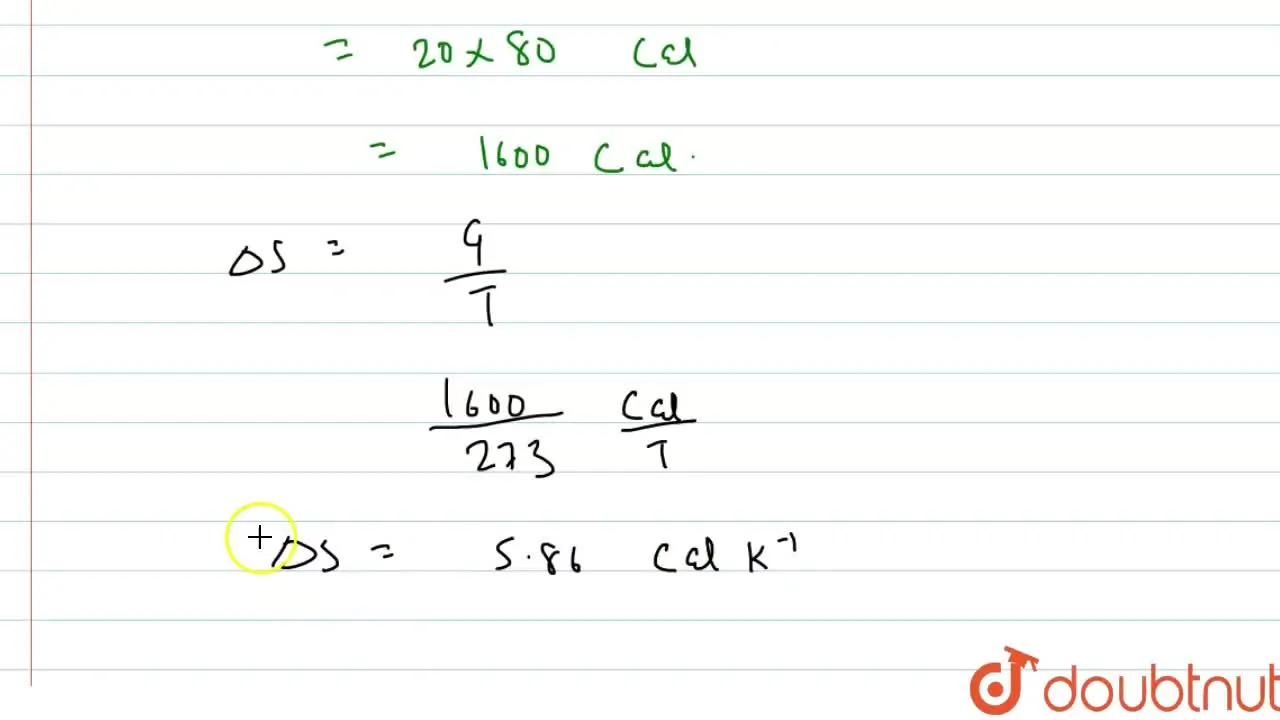
Calculate the entropy change when 20.0 g of ice changes to liquid wate

3) 6025 JAK (4) 602.5 JIK 87. Calculate the entropy change the conversion of 36 g water to vapour 373 K; AH, HO=40.63 k mor! (2) 202.07 JAK () 602 JK (4)
8.What is the entropy change when one mole of ice is converted into water at 0 degree Celsius? (the entropy change for the conversion of ice to liquid water is 6.0 kJ
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)

Latent heat of vaporization as a function of (a) salinity (at 20 °C and

25. The enthalpy of Vaporization of benzere is r.3 kJ/mol its boiling point of suche copy change in the train of Vapour tout its boiling point is ---- 11100 2) +100 B






