the equation of state of a gas is p(v-nb)=rt where b and r are consta - askIITians

the equation of state of a gas is p(v-nb)=rt where b and r are constants. if the pressure and temperature are such that vm=10b what is the value of compressibi
Solved A gas obeys the equation of state p(V-NB) = NkBT with
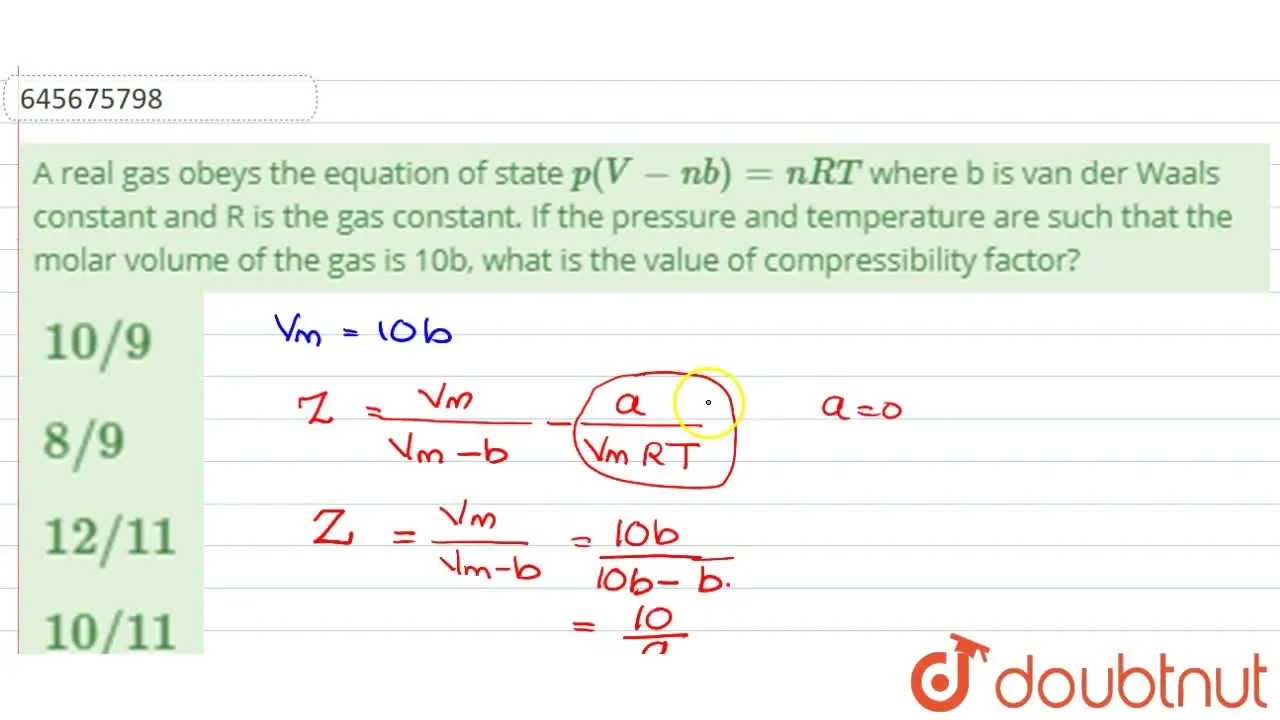
Malayalam] A real gas obeys the equation of state p(V-nb)=nRT where b
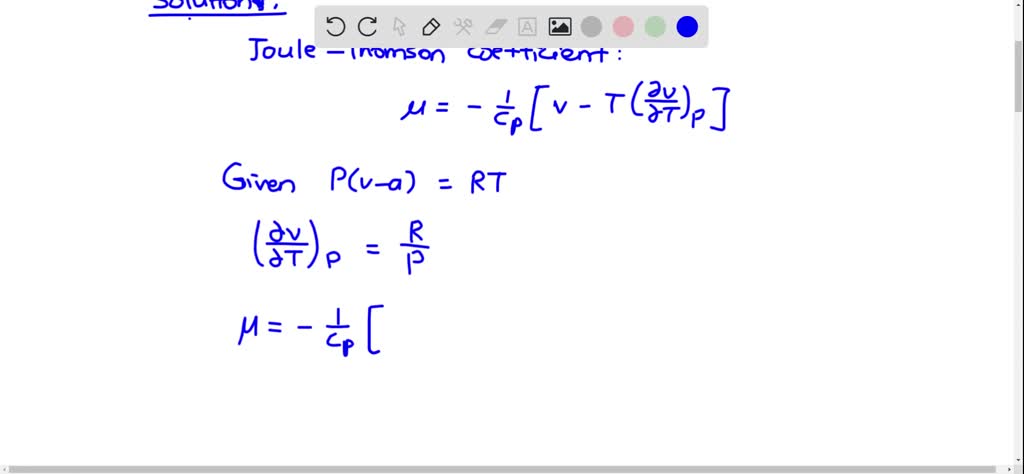
⏩SOLVED:Consider a gas whose equation of state is P(v-a)= R T, where…
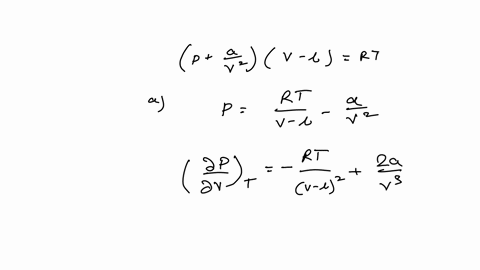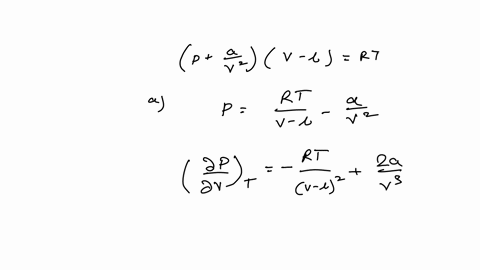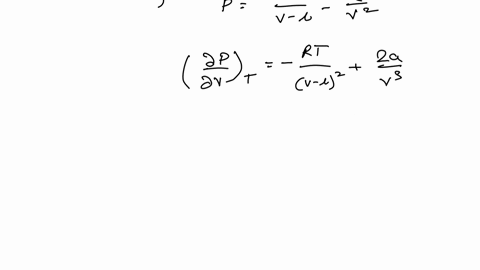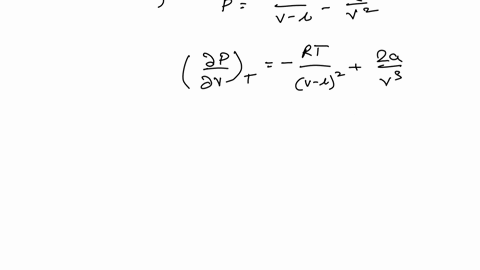
⏩SOLVED:The equation of state of an ideal gas is P V=n R T, where n…
The equation of state for real gas is given by (P+a/V2)(V b)=RT. The dimensions of the constant a and b ??

A gas obeys the equation of state `P(V-b) =RT` (The parameter b is a constnat The

Examples of Chapter ppt video online download
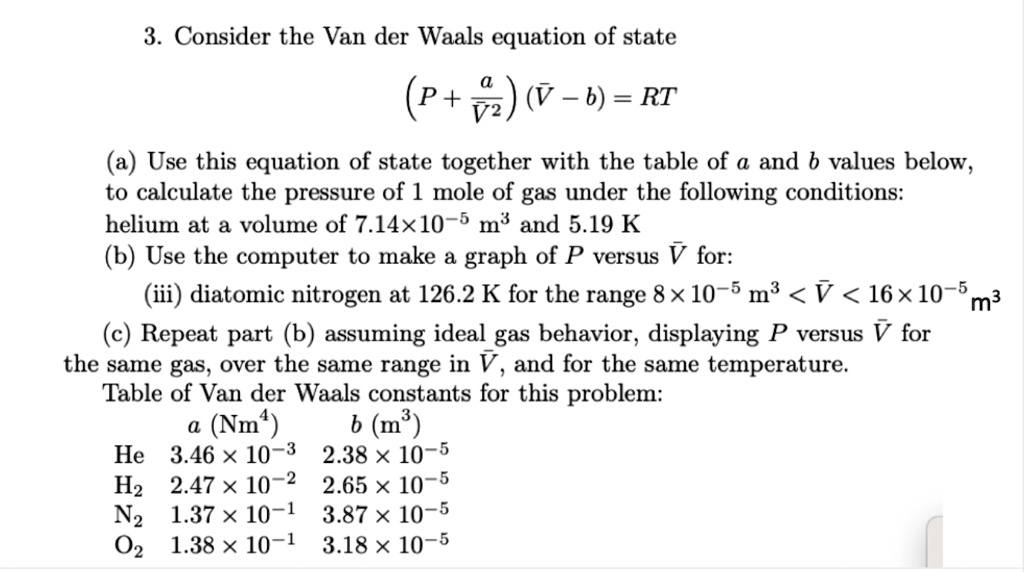
SOLVED: Consider the Van der Waals equation of state (P + a/V^2)(V - b) = RT (a) Use this equation of state together with the table of a and b values below

Tutorials For Chemicalthermodynamics, PDF, Chemical Equilibrium
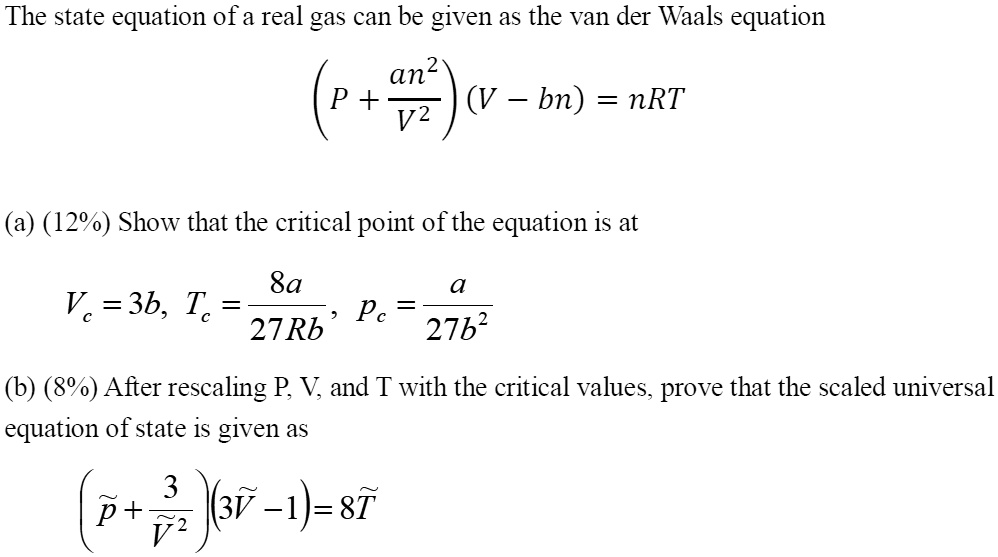
SOLVED: The state equation of a real gas can be given as the van der Waals equation: (P + (an^2/V^2))(V - nb) = nRT Show that the critical point of the equation

SOLVED: The equation of state of a certain gas is given by: P + b = RT. What is the entropy change for an isothermal process? Hint: you can use the first
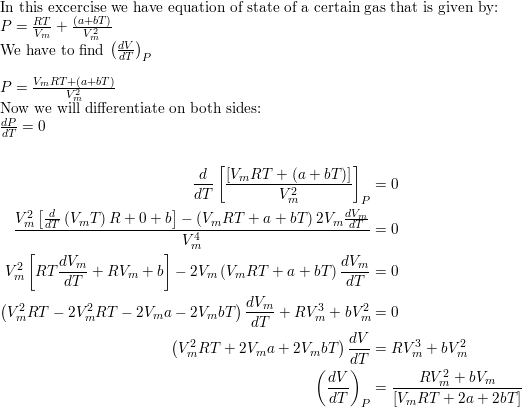
The equation of state of a certain gas is given by $p=R T /

Deviation From Ideal Gas Behavior - Study Material for IIT JEE
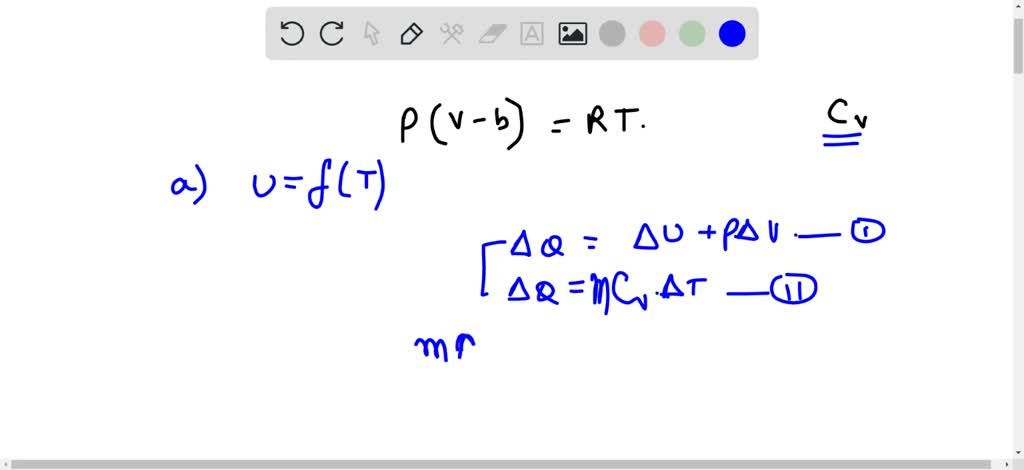
⏩SOLVED:A gas obeys the equation p(V-b)=R T where b is a constant.…

Deviation From Ideal Gas Behavior - Study Material for IIT JEE







